Mass Transfer & Diffusion Coefficient Apparatus Model TH-090
Two separate apparatus, viz Gaseous Diffusion Coefficient Apparatus, involves diffusion with bulk flow & Liquid Diffusion Coefficient apparatus relates to an equi-molar counter diffusion process, are offered to allow measurement of molecular diffusivities. This enables students to familiarize the basic notions of mass transfer theories
TH-091 Gaseous Diffusion Coefficients Apparatus
The diffusion of a vapour ‘A’ from a volatile liquid into another gas ‘B’ can be conveniently
studied by confining a small sample of the liquid in a narrow vertical tube, and observing its rate
of evaporation into a stream of gas ‘B’ passed across the top of the tube. Normally, for simple
instructional purposes, ‘B’ is air and ‘A’ is an organic solvent such as acetone or methyl alcohol.
The apparatus consists essentially of a glass capillary tube placed in a transparent-sided
temperature controlled water bath. A horizontal glass tube is fixed to the upper end of the
capillary tube and air is blown through this by a small air pump included within the unit. This
arrangement allows the maintenance of a partial pressure difference within the capillary tube
between the evaporating liquid surface and the flowing air stream. A travelling microscope, with
sliding vernier scale, is mounted on a rigid stand alongside the thermostatic bath and is used to
measure the rate of fall of the solvent/air meniscus within the capillary
The relation between the measured molar mass transfer rate (‘NA’ per unit area), the partial pressure gradient and the diffusion coefficient D is deduced from the one dimensional steady state
version of Fick’s Law with bulk flow:
Na= -D (CA +CB) / CB dCA/dy
where ‘CA and ‘CB ’are the molar concentrations of the vapour ‘A’ and air ‘B’ respectively
TH-092 Liquid Diffusion Coefficient Apparatus
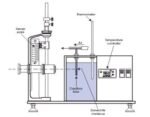
Sci-tech has developed a unique diffusion cell which overcomes the traditional problem of
slow diffusion rates in liquids requiring long observation times, but without sacrificing accuracy or introducing convective effects.
Essentially, the cell consists of a honeycomb of accurately dimensioned capillaries, positioned
between two liquids of differing concentration of the solute whose diffusion coefficient is to be
determined. In practice, a small volume of concentrated solution is placed on one side of the honeycomb, whilst the other side consists initially of a large volume of pure solvent (water). As diffusion of the solute occurs, the concentration within the larger volume increases, and is monitored with a conductivity sensor and meter. The mixture is continuously stirred with a magnetic stirrer to
ensure uniform concentration within the bulk liquid. Whilst the conductivity sensor may be readily calibrated for any required aqueous
system, for introductory studies, dilute solutions of sodium chloride are recommended, for which
conductivity data are provided.
TECHNICAL DETAILS
Diffuser vessel: capacity 1.0 litre
Conductivity meter: 3 ranges 199.9µS to19.99mS
Computer output: RS232
RECOMMENDED ACCESSORIES
Stop clock
Cartridge deionizer
MEASUREMENT AND INSTRUCTIONAL CAPABILITIES
➤ accurate measurement of mass transfer rates in the absence of convective effects
➤ use of Fick’s Law to deduce diffusion coefficients from measurements of mass transfer rate and concentration difference
➤ simple analysis of a first order unsteady state process
➤ effect of concentration on diffusion coefficients
➤ gaining familiarity with the use of laboratory instruments to achieve accurate measurements of
data required for industrial process design
➤ Windows data logging software included
Experimental Capabilities
➤ Accurate measurement of mass transfer rates in the absence of convective effects.
➤ Use of gas laws to calculate concentration differences in terms of partial pressures.
➤ Use of Fick’s Law to deduce diffusion coefficients from measurement of mass transfer rate
and concentration difference.
➤ Simple analysis of a first order unsteady state process.
➤ Effect of concentration on diffusion coefficients.
➤ Gaining familiarity with the use of laboratory instruments to achieve accurate measurements of data required
for industrial process design.
- Software is included to allow the temperature and conductivity in the diffusion vessel to be
displayed, logged and recorded on a customer supplied PC, using a RS232 interface.